 |
The effects of hypoxia on the cell-cycle of normal and cancer cells
The evolution of the cell-cycle is known to be influenced by environmental conditions, including lack of oxygen (hypoxia). Notably, hypoxia appears to have different effects on normal and cancer cells. Whereas both experience hypoxia-induced arrest of the G1 phase, experimental evidence suggests that only cancer cells undergo hypoxia-induced quiescence.
We develop a cell-cycle model to account for the action of the protein p27. This protein, whose expression is upregulated under hypoxia, inhibits the activation of the cyclin dependent kinases, thus preventing DNA synthesis and delaying normal progression through the cell-cycle. Using a combination of numerical and analytical techniques to study our model (a coupled system of nonlinear ordinary differential equations), we have tested the model-driven hypothesis that the difference between the behaviour of cancer and normal cells under hypoxic conditions is due to differences in the size-regulated production of p27. We have shown that such a model is consistent with a number of experimental observations. See publication 169.
 |
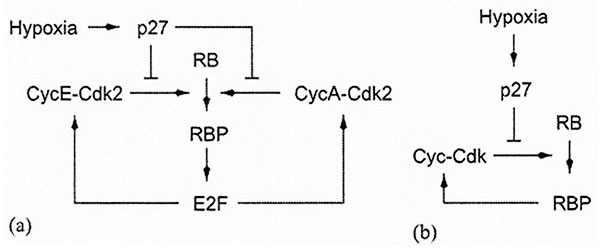
Schematic representation of the mechanism for hypoxia-induced cell-cycle arrest in normal cells (fibroblasts). (a) The original mechanism proposed by Gardener et al, Hypoxia Inhibits G1/S transition through regulation of p27 Expression, J. Biol. Chem., (2001). 276, 7919-7926. (b) Our simplified version. RBP stands for the phosphorylated form of the RB protein. [Reproduced with permission from publication 169].
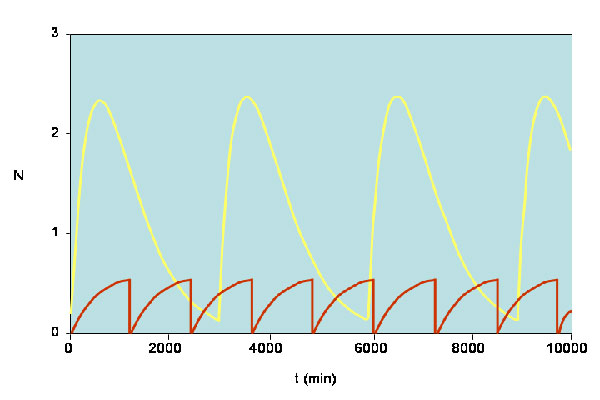 |
Diagram showing how levels of p27 expression vary for the normal and cancer cells. The yellow line corresponds to normal cells whereas the red line corresponds to cancer cells. These results agree with the observation that low expression of p27 is a poor prognostic indicator. [Reproduced with permission from publication 169].
** Work carried out in collaboration with T. Alarcon and H.M. Byrne