I am interested in the mathematical modelling of fluid mechanical, industrial and medical problems. Active research areas include Photovoltaic devices, Surfactant systems, Tear films, Industrial mathematics and Mathematical modelling in medicine and biology. |
Surfactant systems
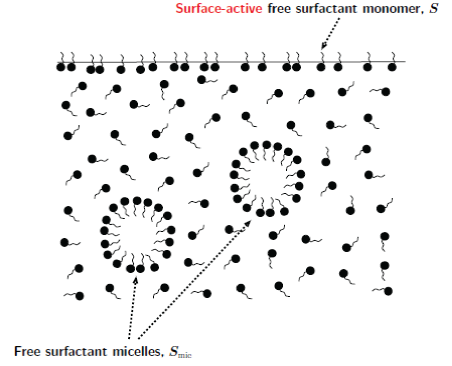
Surfactants are molecules that preferentially reside at air-liquid
interfaces, where they reduce the surface tension. Non-uniform adsorption of surfactant generates a gradient in surface tension which can cause a
fluid to flow. Above a certain critical concentration, it is also
energetically favourable for surfactant molecules to form aggregates
called micelles in the bulk liquid. Some surfactants dissociate into ion
pairs when dissolved in water.
Over the past 15 years we have studied numerous surfactant problems.
The overflowing cylinder experiment
 |
This experiment is used to characterise surfactant adsorption onto a continuously expanding interface. Liquid is passed up within a cylinder, forms a flat free surface and then flows down the outside of the cylinder, where it is recirculated. The introduction of a small amount of surfactant results in an increase in the velocity at the air-liquid interface of up to a factor of ten. Our mathematical model for the overflowing cylinder shows that the weir flow at the edge of the cylinder is crucial for determining the concentration at the centre, and our theoretical results match well with the experimental results of our collaborator, Colin Bain and his group (now in Durham). |
|
Related publications: Macroscopic modelling of the surface tension of polymer-surfactant systems |
Micellar dynamics
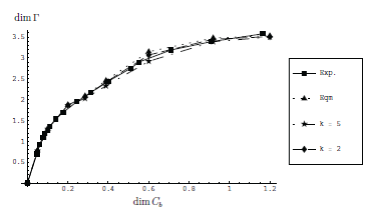
| We have extended our "straining flow" model for the overflowing cylinder to include a simple reaction which makes micelles of a given (single) size. |
|
| Our model can be used to determine the rate constants for the formation and dissociation of micelles, as shown in this figure: |
 |
Related publications: |
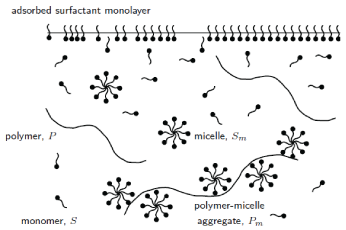
Polymer-surfactant mixtures and multilayer structures at interfaces
We have an ongoing collaboration with the Rutherford Appleton Laboratory, examining the behaviour of polymer-surfactant mixtures. Some of these mixtures, known as strongly-interacting mixtures, exhibit unexpected surface tension profiles:
|
 |
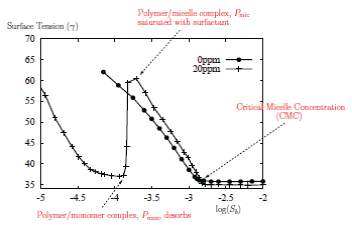 |
We have developed a model which is able to accurately predict where the surface tension starts to increase, and this is being used by chemists to understand these systems. We have also examined the distribution of polymer-surfactant solutions in a straining flow. Our current work is looking at the formation of multilayer structures at interfaces. Related publications: |
Ionic surfactants
We have had a fresh look at the old problem of the adsorption of ionic surfactant at an air-liquid interface. Standard drift/diffusion models predict too high a concentration of counter ions just below the surface, and several fudges exist to counter this. We have postulated a new model, where surfactant ions recombine in the bulk as well as near the surface, which negates the need for a fudge.
|
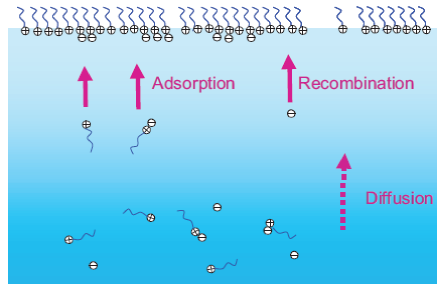 |